Author(s): Sumita Subarno
Paper Details: Volume 3, Issue 3
Citation: IJLSSS 3(3) 19
Page No: 271 – 289
ABSTRACT
The overlap between the IPR and public health is an issue of global concern, especially concerning traditional medicine access in India and other Asian countries. Patent infringement laid out here includes the right to work a patented invention without the patent holder’s authorization, and further allows governments to grant authorizations to produce under patent to those violating patents under the conditions specified in that statute – a de facto use of patents for public health. Since 1996 when the World Health Organization (WHO[1]) discussed the consequences of the TRIPS Agreement, developed following the signing of the Uruguay Round within the WTO. Traditional medicine-based traditional treatments, developed without patenting, are not readily available through pharmaceutical corporations and are not accessible in resource-limited settings. India’s patent regime, using TRIPS flexibilities is seen among other Asian nations for demonstrating its boundaries, offering a continuous use of compulsory licensing for drugs to be an exemplar of prioritising public health.
The existing models of compulsory licenses have promoted commercial interests more than the human rights, have not been able to protect the intellectual heritage of indigenous peoples and have not been able to connect the trade economics to access to medicaments. They do not have strong benefit-sharing and indigenous rights provisions in those. Reforms need to integrate moral rights principles, protect traditional knowledge, secure equitable returns for indigenous communities, promote effective benefit-sharing and increase access to core traditional medicines.
The study will also look at challenges to implementation, such as resistance from pharmaceutical companies, regulatory barriers, and international trade issues and aims to suggest a better approach that balances the interests of patent holders, indigenous communities, public health needs, and international trade obligations, ensuring fair access to traditional medicines while protecting cultural and intellectual heritage with the of legal mechanism of compulsory licensing.
KEYWORDS: Biopiracy, Compulsory Licensing, Public Health, TRIPS Agreement, Traditional Medicines, Traditional Knowledge.
INTRODUCTION
The relationship between intellectual property rights and public health has been a long-held debate globally without any suitable framework to fill the default void of these two concepts, more particularly in the context of traditional medicines. With increasing focus on patents in the pharmaceutical sector, the enforcement of IP rights can operate as an obstacle to access for the communities which depend exclusively on traditional health care. Compulsory licensing, a legal provision that permits governments to license the manufacture of patented drugs without the patent holder’s consent under defined conditions, is an increasingly important tool for securing access to affordable medications. Moreover, in India and a number of other Asian countries, compulsory licenses act as a conduit between patent enforcement and public health interests, all the more so as traditional medicines significantly contribute towards healthcare.
The issue of intellectual property, public health, and access to medicines emerged in the World Health Organization (WHO) for the first time in 1996. The timing practically coincided with the end of the Uruguay Round and with the creation of the World Trade Organization (WTO).[2] In 1995, the University of Madrid Carlos Tercero organized a conference together with the WHO Essential Medicines Programme, where Professor Carlos Correa presented a paper entitled “The Uruguay Round and Drugs.” The paper analyses the possible implications of the TRIPS agreement on access to medicines and reveals the “margin of manoeuvre” that the Agreement has to protect Public Health. “The Uruguay Round and Drugs” is the first document that specifically alerts the health sector to the possible implications of the TRIPS agreement on public health and, more specifically, on access to medicines[3]. Traditional medicines, deeply embedded in cultural and historical practices, have gained increasing recognition in global healthcare. Although, pharmaceutical companies have been filing patents on formulations taken from traditional knowledge; the commercialization strategies adopted and also the limited access for years together to these drugs have steamed up ethical and legal quandaries. The Compulsory licensing tool provides an avenue to reconcile the conflicting important interests of intellectual property rights and public health by ensuring access to traditional medicines on a fair and equitable basis in poor resource countries.
India’s patent regime, influenced largely by the TRIPS Agreement and its flexibilities, has established important principles for the use of compulsory licencing for essential drugs. Other Asian countries have also been investigating similar mechanisms for safeguarding the public health by maintaining intellectual property standards. In reviewing the working of the mechanisms of compulsory licensing in cross-border trade of traditional medicines, the thesis has sought to evaluate the potential of such mechanisms to promote access and prevent indigenous knowledge from being exploited through monopolistic means.
This study examines case studies, legislative and policy interventions in India and Asia, to ascertain how the tool of compulsory licensing can be used to shape fair healthcare. It will also examine implementation challenges, such as resistance from drugmakers, regulatory obstacles, and the impact on international trade. And eventually, this theory aspires to offer a holistic view on how compulsory licensing can fill in the contradiction between patent enforcement and public health in the medicinal trafficking of traditional drugs.
SIGNIFICANCE OF THE STUDY
This study has also illuminated the significant role of compulsory licences in the pharmaceutical trade in traditional medicines in India and Asia, which is relatively less researched compared to orthodox pharmaceuticals. It speaks to the urgent tension between the enforcement of patents and the public health requirements of the poor, and in particular, the least powerful in society within traditional knowledge systems, such as Ayurvedic, Unani, Siddha, and Traditional Chinese medicine. Compulsory licensing is examined as a legal tool to encourage the affordable availability of medicines, guard traditional knowledge, and bring about fair benefit sharing.
Academically, this is a highly relevant contribution to the field, as it constitutes an interdisciplinary as well as comparative study between intellectual property law, public health, and indigenous studies. Through a study of the regimes in India, China, Thailand, and ASEAN nations, and concerning specific cases such as India’s compulsory licensing of Bayer’s Nexavar[4], the article contributes to the academic debate and provides a platform for future research on traditional medicine-derived pharmaceuticals.
On a policy level, the study offers pragmatic solutions to improve current systems of compulsory licenses, coming up with effective responses to issues, for instance, biopiracy and access hurdles. It provides an orientation not only for national policy makers but also for IOs such as WHO, WIPO and WTO, stressing the need for TRIPS flexibilities to be tailored to traditional medical cultures. On the social front, the research supports health rights of low-income populations and the conservation and ethical commercialization of indigenous knowledge, referencing tools such as India’s Traditional Knowledge Digital Library (TKDL)[5].
Its broad content is consistent with international efforts, such as the WHO’s Traditional Medicine Strategy 2014–2033[6] and the Nagoya Protocol, that demonstrate India’s capacity to lead the way in using flexibilities in laws to address public health. In the end, this study provides an overall model for advancing a just, accessible, and culturally respectful pharmaceutical system for traditional medicines in the Global South.
STATEMENT OF PROBLEM
This study examines the tension between intellectual property rights (IPR) enforcement and public health goals in India and Asia, with a particular focus on traditional medicines. It illustrates how the patent protection as in the context of TRIPS, for example – condones that an emphasis on pharma profits trumps the need for affordable traditional medicines to marginalised communities. Patents on traditional knowledge can cause prices to inflate and availability to drop, while indigenous knowledge is rarely protected and may be exploited for biopiracy without reward.
Compulsory licensing mechanism can fill this gap, but it has not been much used or extended to traditional medicines because of unclear guidelines, industry lobbying and international trade compulsions. Furthermore, existing systems do not include appropriate benefit-sharing processes, which results in marginalisation of indigenous peoples both economically and culturally. This study suggests that current forms of compulsory licensing could be more useful, but are structurally inadequate in responding to access, knowledge protection, trade distortions and fairness. It proposes a reformed, rights-based approach to compulsory licensing or its equivalent, which would be conducive to fair access, the protection of traditional knowledge and beneficial resource sharing, and to the advancement of health equity and social justice.
RESEARCH QUESTIONS
1. How effectively do current compulsory licensing frameworks protect both patent holders’ rights and public health interests in traditional medicine?
2. What are the key challenges in implementing compulsory licensing for traditional medicine-based pharmaceuticals in India and Asia?
3. What role do international trade agreements play in shaping compulsory licensing policies for traditional medicines?
4. How can benefit-sharing mechanisms be improved to ensure equitable distribution of traditional medicine benefits?
BACKGROUND OF TRADITIONAL MEDICINES IN INTELLECTUAL PROPERTY RIGHTS
Traditional medicines, such as Ayurveda, Traditional Chinese Medicine (TCM[7]), Unani, Siddha, Thai herbal medicine, etc., are the bedrock of healthcare in many Asian countries and have been in use for several centuries for India and thousands of years for China. These systems are based on biodiversity, are plant, mineral, animal based and are deeply ingrained within cultural and spiritual heritage. The knowledge behind these medicines is usually held communally and is transmitted orally, for example in the form of traditional songs, maintained through oral tradition, or shared via certain texts, such as the Ayurveda or certainly the Chinese medicine. Traditional medicines have become the topic of commercial interest with the globalization and development of the pharmaceutical industry, and they are being incorporated into modern healthcare systems and markets.
Traditional medicine has been the great heritage of mankind, but the scenario has changed altogether with the introduction of intellectual property right (IPR) particularly patents. [8]The Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement, which was established by the World Trade Organization (WTO) in 1994, requires patents to be granted for new, useful, and non-obvious pharmaceutical products. Under this system, pharmaceutical enterprises have been able to patent compounds based on traditional knowledge because these companies have usually not attributed the derived formulations to nor compensated the indigenous people who discovered it. These are potentially illegitimate and excoriated as biopiracy, as illustrated by turmeric and neem patents in the United States and other patents awarded in India and subsequently revoked after India proved history of traditional use. In a bid to combat biopiracy, India has set up a database of traditional knowledge from medicine systems known as The Traditional Knowledge Digital Library[9] to prevent the granting of a patent on already known medicinal knowledge, for which The Council of Scientific and Industrial Research has received praise. Similarly, China has enacted measures that safeguard TCM, such as patent regulations that acknowledge traditional mixtures. Other Asian countries, like Thailand and Malaysia, have also established protection frameworks for their herbal medicine systems, with varying success. The balancing of protection of TK with need to ensure availability of affordable medicines also made it necessary for the TRIPS flexible instrument of Compulsory Licensing (allowing governments to authorize generic production of patented medicines without the consent of the patent-holder) a necessity[10]. In India, compulsory licensing has been used to enhance access to essential drugs, as seen in the Natco Pharma v. Bayer[11] case (2012), which involved the cancer drug Nexavar.
The interface of traditional medicaments and IPR poses difficult ethical, legal, and economic issues. While patents encourage innovation, they can block access to medicines, especially in poor communities that still depend on traditional cures. Compulsory license being one of the ways of reconciling the rights of the patent holders with the public health concerns could be the answer, but the application of this mechanism with regard to traditional medicine has not been fully explored, hence the need for more research on the effectiveness and limits of compulsory license.
CHALLENGES OF TRADITIONAL MEDICINES IN IPR REGIMES
This study examines the vexed interdependence of patenting rights and the pharmaceutical commerce in traditional medicines as it operates in India and in Asia more generally, where the developmental impacts of intellectual property law are encountered in the commerce, availability, and preservation of traditional remedies. Traditional Medicines, which include systems similar as Ayurveda, Unani, Siddha, Traditional Chinese Medicine (TCM) along with other herbal traditions, are grounded on age-old knowledge systems, which were always and continue to be considered non-commercial and community wise. Nevertheless, the modern patent systems, in particular after the execution of the Trade-Related Aspects of Intellectual Property Rights (TRIPS) Agreement[12] in 1995, have turned these drugs into goods that can be vended on the request, with important legal, moral, and profitable implications.
Patenting rights allow pharmaceutical companies to monopolise the manufacture and trade of traditional medicine derived fusions of the formulas, often increasing prices and controlling access to low-income users who consider traditional medicine as a primary health service. For circumstances, when companies patent derivations of traditional knowledge such as formulations based on turmeric, neem, or ginseng they can monopolize the market, sidelining traditional practitioners and users. This business has sparked debates about the appropriateness of patenting knowledge that is collective, culturally significant, and frequently undocumented, as it risks indigenous communities from their own heritage rights.
The section examines crucial pivotal of biopiracy, where traditional knowledge was patented without acknowledgment or compensation to indigenous communities. proved instances include the U.S. patents on turmeric for injury recovery and neem for antifungal parcels, both of which were thereafter repealed after India’s intervention, addressing the vulnerabilities of traditional knowledge in global patent systems. These cases emphasize the need for mechanisms like India’s Traditional Knowledge Digital Library( TKDL), which has been demonstrating traditional drugs knowledge to prevent misappropriation by securing it has honoured as former art, therefore ineligible for patenting.
The legal frameworks governing patenting in India and Asia. In India, the Patents Act, 1970, as amended to conform with TRIPS, allows patents for novel, nonobvious, and industrially applicable inventions but includes provisions to cover traditional knowledge. Section 3( p) of the Act excludes from patentability[13] “ an invention which, in effect, is traditional knowledge or which is an aggregation or duplication of known parcels of traditionally known ingredients,” furnishing a safeguard against biopiracy. nevertheless, inscrutability in defining “ novelty ” and “inventive step ” for traditional drug components frequently leads to legal disputes, as seen in cases involving Ayurvedic or TCM-formulation medicines. China, has developed a strong patent system for TCM, integrating traditional knowledge into its public health framework while allowing patents for new elements or processes. Thailand and other ASEAN nations, meanwhile, looking forward various challenges in the implementation of patent laws with traditional medicine practices, often providing limited protections for communities with indigenous knowledge. These varying approaches impact the pharmaceutical trade, particularly in terms of availability, pricing, and invention.
TRIPS AGREEMENT, PUBLIC HEALTH, AND OTHER RELATED TREATIES
This section discusses the Trade- Related Aspects of Intellectual Property Rights( TRIPS) agreement in depth, including its implications for public health and relationship with other multinational treaties in the medium of traditional medicinal trade. Coming into force in 1995 as part of the World Trade Organization (WTO) rules, the TRIPS Agreement established international standards for intellectual property protection. The agreement, for example, requires all member states to provide patents for 20 years from the date of application for inventions that are novel, inventive, and capable of industrial applicability[14]. This presented a stronger patent regime for the pharmaceutical companies, as medicine costs escalated, including medicinal derived from traditional knowledge.
Among the many implications, this presents serious challenges to public health in developing countries, such as India or other Asian countries .Understanding the TRIPS Agreement implications for public health may have started around the late 1990s, around the time of the WHO discussions in 1996 and the paper written by Carlos Correa in 1995[15], “The Uruguay Round and Drugs[16] ”. Correa was perhaps the first to bring to the attention of the health sector the implications of the TRIPS Agreement in strengthening patent monopolies and thereby restricting access to medicines. This concern eventually led to the 2001 Doha Declaration on the TRIPS Agreement and Public Health, confirming that TRIPS shall not prevent member states from protecting public health. The Declaration underscored flexibilities related to compulsory licensing, parallel imports, and exemptions for least-developed countries provided governments the leeway to prioritize affordable access to essential medicines. With regard to traditional medicines, the flexibilities offered through TRIPS have been important for countries like India.
In 2012, India executed its first compulsory license for Bayer’s cancer drug Nexavar meaning that the pricing for treatment was significantly lowered. However, the realities of utilizing flexibilities through compulsory licensing and others with respect to traditional medicines is far more complicated because traditional knowledge often does not meet the formal criteria it deserves, in a way that is appropriate for patent eligibility. The section examines how India was able to employ TRIPS flexibilities to protect public health using Section 3(d)[17] of the Patents Act that addresses ‘evergreening’ patents by also requiring enhanced efficacy for the new forms of known substances; this was also applied to traditional medicine formulations. Other Asian countries like Thailand and Malaysia also used compulsory licensing to satisfy public health concerns without sufficient application to traditional medicines. The section evaluates the differences as well as describes how international trade pressures resulted from developed nations and pharmaceutical companies push back against using TRIPS flexibilities in the form of bilateral trade agreements or action in the WTO[18].
This section went beyond TRIPS to mention other treaties that may impact trade in traditional medicine. The Convention on Biological Diversity (CBD[19]) and Nagoya Protocol (2010) discuss access and sharing of benefits (ABS) of genetic resources and related traditional knowledge, and call for fair sharing of the benefits from the commercialisation of the genetic resources/related traditional knowledge[20]. These treaties are necessary to protect the rights of indigenous communities related to traditional medicines, but the end implementation may be problematic given limited enforcement capacity and the fact that countries may take different legal approaches to the treaties.
Further, World Intellectual Property Organization (WIPO[21])’s ongoing negotiations of genetic resources, traditional knowledge and folklore seek to establish a global framework for the protection of the knowledge of traditional medicines; however, a global framework is still slow to emerge. TRIPS conventions and other treaties also act on the pharmaceutical trade of traditional medicines, like a double-edged sword – they can work to strengthen patent protection as well as provide the flexibility to prioritise public health. We must find a way to integrate public health and indigenous rights principles into these treaties and processes to provide adequacy of access to and protection of traditional medicines.
COMPULSORY LICENSING IN INDIA AND OTHER ASIAN COUNTRIES: STATUTORY PROVISIONS, JUDICIAL INTERPRETATION AND CASE STUDIES
In India and across Asian countries, compulsory licensing frameworks vary significantly, reflecting diverse legal traditions, economic priorities, and public health needs. Countries like Thailand, China, and Malaysia have incorporated compulsory licensing provisions, often inspired by TRIPS flexibilities, to ensure public health needs , including access to traditional medicines such as Traditional Chinese Medicine (TCM), Thai herbal remedies, and Malaysian ethnobotanical formulations. These legal frameworks share a common goal with India’s system to balance patent rights with public welfare but differ in their statutory scope, implementation mechanisms, and judicial oversight, particularly concerning traditional medicines.
A possible balance between public health and patent rights is a key component of India’s compulsory licensing system, which was amended in 2005 to conform to TRIPS. This is especially focused upon traditional medicines like Ayurveda. Section 84 of the Act[22] allows compulsory licenses for three years following the patent’s issuance in the event that an invention is not affordable or meets public needs. Section 92 of the Act[23] permits government to issue licenses for national emergencies or public non-commercial use, bypassing the waiting period. These provisions prioritize access to essential medicines including traditional medicines as well. Judicially, Bayer Corporation v Natco Pharma Ltd (2012) granted India’s first compulsory license for Nexavar which a formula for cancer drug, citing the reasons upon high costs and limited availability, upheld by the IPAB and Bombay High Court, emphasizing local manufacturing and affordability. This precedent applies to traditional medicines that have restrictive patents. Although they are not directly related to compulsory licensing, case studies such as the patent challenges for neem and turmeric through the Traditional Knowledge Digital Library (TKDL) emphasize the prevention of biopiracy. Tensions in patenting traditional knowledge are highlighted by the rejection of Ayurvedic patents based on ancient texts. [24]The use of compulsory licensing for traditional medicines is restricted; in order to guarantee fair access and preserve indigenous knowledge, more precise regulations and judicial attention are required.
The Thai Patent Act,1976[25] as amended, governs compulsory licensing in Thailand and permits the government to grant licenses for pharmaceuticals that are patented in situations where the patent is not functional or public health demands it. When Thailand granted mandatory licenses for antiretroviral medications (such as efavirenz and lopinavir/ritonavir) and the medication for heart disease, clopidogrel, between 2006 and 2008, it garnered international attention. The increase incidence of HIV/AIDS and the high cost of patented medications were cited as public health justifications for these licenses. Although the focus of these cases was on contemporary pharmaceuticals, Thailand’s approach is pertinent to traditional medicines because the nation has a long history of herbal remedies which are recorded in books such as the Tamra Phra Osoth. Government has explored compulsory licensing to prevent biopiracy and ensure access to patented herbal formulations, but statutory provisions lack specific guidelines for traditional medicines, and judicial interpretations remain sparse due to limited litigation in this area.
Traditional Chinese Medicine, China has implemented compulsory licensing under the People’s Republic of China’s 1984 Patent Law, which was revised in 2020. While Article 50[26] permits licenses for patented drugs to address public health needs, Article 49[27] permits compulsory licenses in cases of national emergency or public interest. Although both domestic and foreign entities have patented TCM formulations worldwide, China has taken a cautious approach and has not seen any notable instances of compulsory licensing for TCM. In China, courts have rarely addressed compulsory licensing disputes, indicating a preference for negotiation over litigation, and judicial interpretations frequently prioritize state interests. Case studies, however, like the patenting of antimalarial medications based on artemisinin and originating from TCM, emphasize the potential for compulsory licensing to ensure access to traditional medicine-based drugs, particularly in global health crises.
Malaysia, where the Patents Act 1983 governs compulsory licensing and permits licenses in situations involving non-working patents, public health emergencies, or anti-competitive behaviour under Part X (Compulsory Licences) Section 49 to 54 of Patents Acts 1983[28]. An important step in utilizing TRIPS flexibilities was taken in 2017 when Malaysia granted a compulsory license for the hepatitis C medication sofosbuvir. Biopiracy poses a threat to Malaysia’s rich biodiversity and ethnobotanical knowledge, especially among indigenous communities, which are used in traditional medicines. The government has initiated compulsory licensing as a way to safeguard traditional remedies, but there are limited judicial precedents and very broad and ambiguous statutory provisions. Malaysia to mitigate the possible balance between patent rights and granting the public access to traditional medicines has been demonstrated by case studies, such as the patenting of formulations of tongkat ali (Eurycoma longifolia[29]) commonly used in traditional ‘antiaging’ treatments to address decreased energy, mood, libido and hormonal imbalances.
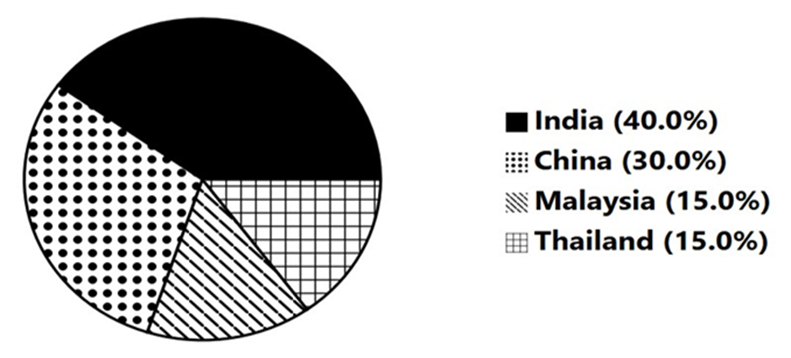
Figure 1: Comparative Efforts to Prevent Biopiracy in Traditional Medicine.
India (40%) leads due to initiatives like the Traditional Knowledge Digital Library (TKDL), and active legal interventions (e.g., neem, turmeric patent reversals). China (30%) follows closely with structured Traditional Chinese Medicine databases and integration of TCM in the patenting system. Malaysia and Thailand (15% each) have made moderate progress, primarily through biodiversity and heritage protection laws and local digital registries.
A comparative analysis of these frameworks suggests that Asian countries could benefit from harmonizing their approaches, drawing lessons from India’s more robust judicial and statutory framework while amending provisions to address the cultural and economic significance of traditional medicines .
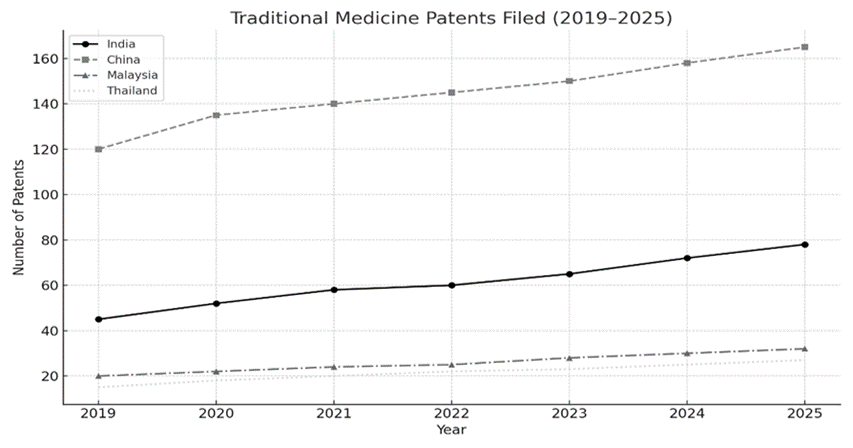
Figure 2: Patent Trends in Traditional Medicine (2019–2025)
In conclusion, all jurisdictions have a common shortcoming when it comes to traditional medicines, even though India’s mandatory licensing system is more developed than those in Thailand, China, and Malaysia. The need for reform is underscored by the absence of benefit-sharing mechanisms, limited judicial engagement, insufficient protection of indigenous knowledge, and a lack of specific provisions.
MEDICINE ACCESSIBILITY AND PRICING DYNAMICS
The availability of traditional medicines such as Ayurveda, Traditional Chinese Medicine (TCM), and Thai herbal remedies is a critical public health concern in India and other Asian countries where these systems play an important role in healthcare delivery, particularly for underserved groups. Compulsory licensing, as a medium for overcoming patent restrictions, is critical in improved access by allowing the production of low-cost generic versions of patented medicines. However, the impact on traditional medicines is complicated, given the unique challenges of patenting traditional knowledge and the cultural significance of these remedies.
Compulsory licensing enhances access to drugs by eliminating obstacles created by increased prices and restricted availability, often stemming from patent monopolies. The notable case of Bayer Corporation v. Natco Pharma Ltd. (2012) in India showed how compulsory licensing can enhance access to vital medicines. The Indian Patent Office improved the accessibility of Bayer’s cancer drug Nexavar by allowing the government-assigned company Natco to produce a generic variant to reduce the monthly price from around Rs 2.8 lakhs to Rs 8,880 so as to meet public needs[30]. While this situation pertains to a modern pharmaceutical, the principle extends to traditional medicines, where access to herbal remedies might be restricted by patents. For instance, if a global company secures a patent on an Ayurvedic formulation derived from traditional knowledge, the elevated costs could deter rural populations from utilizing these treatments for extended periods.
Pricing dynamics are intricately linked to accessibility, as the price of medicines and medications significantly affects their affordability for low-income developing country communities. The literature available emphasizes that compulsory licensing in the herbal medicine sector can reduce prices by encouraging competition among generic producers, which will work as a healthy competitive market among the local producers as well. In Thailand, the granting of compulsory licenses for antiretroviral medications from 2006 to 2008 decreased expenses by as much as 90%, allowing the government to enhance HIV treatment initiatives. Corresponding method can be exercised for traditional drugs, where patented creations frequently attract higher prices because of their interpreted effectiveness and global necessity. Monetization of Traditional Chinese Medicines derived medicines such as artemisinin for malaria has resulted in increased costs in global markets, which has restricted access in developing nations. Compulsory licensing could mitigate this by the production of these medicines are processed locally at lower cost, henceforth, helping both regional and global communities. Unlike contemporary pharmaceuticals, traditional medicines frequently lack standardized formulations, making it problematic to define the extent of a compulsory license. Additionally, the patenting of traditional knowledge is frequently contested on grounds of former art, as seen in India’s use of the Traditional Knowledge Digital Library (TKDL) to challenge patents on turmeric and neem. While the TKDL reviews on misappropriation, it does not directly address access to patented formulations that are legitimately granted. Compulsory licensing could bridge this gap, but its underutilization in the traditional medicinal sector due to regulatory inscrutability and opposition from pharmaceutical companies limits its effect. Further, the pricing benefits of compulsory licensing may be unevenly allocated, as generic manufacturers may prioritize urban markets over rustic areas, where traditional medicinal are most necessitated.
Thus, compulsory licensing has the capability to significantly enhance medicinal accessibility and appropriate improved pricing dynamics for traditional medicines in India and Asia. As documented by medicines like India’s Nexavar case and Thailand’s antiretroviral licenses, policy administrative can develop strategic approach to ensure that patented traditional medicines remain reasonable and globally available. However, addressing the unique characteristics of traditional medicines, such as their cultural significance and variable formulations, requires amended policies to maximize the public health benefits of compulsory licensing.
POLICY RECOMMENDATION
To promote fair trade in traditional medicines India and Asian countries should take leverage of TRIPS flexibilities through they can incorporate to simplify compulsory licensing processes and creating robust reviewing systems. For instance, administrator can authorize a dedicated task force like India’s Traditional Knowledge Digital Library to prevent biopiracy and protect indigenous knowledge. Policies should incorporate fair benefit-sharing agreements that include royalties, technology transfers, or community development funds. Active encouragement to regional cooperation through initiatives like an ASEAN-led traditional knowledge database to prevent misappropriation.
In trade debates , these countries should strongly oppose TRIPS-plus provisions that limit access to medicines. And instead, they should try to implement measures that focus on public health and indigenous rights. Furthermore, governments should support research and development with public funding and partnerships that involve traditional healers. This will help keep innovations affordable and culturally relevant. International trade frameworks need to have monitoring systems, like WTO-led impact assessments, to see how intellectual property rules affect access to medicines and suggest practical changes. Lastly, capacity-building programs, backed by organizations like WIPO, should enable indigenous communities to take part in policy discussions and negotiate fair benefit-sharing deals. Together, these actions would create a trade environment that balances commercial interests with public health, cultural preservation, and sustainable economic growth, making Asia a leader in the ethical trade of traditional medicines.
Additionally, we should suggest key policy changes to improve compulsory licensing frameworks for traditional medicines in India and Asia. First, national patent laws should clearly state that traditional medicines can qualify for compulsory licensing. India’s Patents Act could lead this change by focusing on herbal treatments for chronic diseases. Second, mandatory benefit-sharing systems should follow the Nagoya Protocol, ensuring that indigenous communities receive royalties, profit shares, or trust-fund investments, supported by national Traditional Knowledge registries. Third, ASEAN should lead regional efforts by standardizing compulsory licensing procedures, recognizing systems like India’s TKDL, and promoting joint research to validate traditional medicines. Fourth, capacity-building programs must educate officials and empower indigenous communities on IP rights, along with public health campaigns that encourage affordable access. Fifthly, Asian nations need to work together to resist TRIPS-plus pressures through WTO coalitions, seeking exemptions for traditional medicines and amendment to TRIPS that could recognize traditional knowledge And India should take initiate in this effort through its capability of drawing on its advocacy experience.
Finally, compulsory licensing should combine with public health systems through production subsidies, quality control, and infrastructure investments, especially in rural areas, to ensure fair access. These measures would create a balance between IP protection, cultural preservation, and healthcare access, making Asia a global example of ethical traditional medicine trade.
CONCLUSION
To summarize this study which underscores the critical role of compulsory licensing in reconciling intellectual property rights (IPR) with public health imperatives, particularly in the context of traditional medicines in India and Asia. While compulsory licensing has robust proven effective in enhancing access to modern pharmaceuticals as documented by India’s landmark case Natco Pharma v. Bayer its application to traditional medicines remains underexplored and underutilized . This study emphasis upon systemic gaps, including the lack of unambiguous legal provisions for traditional medicines, no explicit benefit-sharing mechanisms for indigenous communities, and regulatory ambiguities that hinder equitable access and it also reveals that current frameworks prioritize monitory interests over human rights and cultural preservation, often marginalizing indigenous knowledge holders. However, strategic reforms can address these gaps. And looking forward strengthening of TRIPS flexibilities, mandating benefit-sharing agreements modelled on the Nagoya Protocol, and establishing regional databases (e.g., an ASEAN-wide traditional knowledge repository) would prevent biopiracy while ensuring fair compensation. Judicial activism, as seen in India’s revocation of turmeric and neem patents, should be backed with legislative clarity to define the scope of compulsory licensing for traditional medicine formulations. Moreover, International cooperation among the countries is essential to counter TRIPS-plus pressures and harmonize policies. Thailand and China offer valuable lessons in leveraging compulsory licensing for public health, but countering these still a unified approach is needed to address pricing disparities and ensure rural access. Policymakers must also encourage incentivize research and development in traditional medicines through public-private partnerships, aligning innovation with affordability.
Lastly, this study advocates for a rights-based overhaul of compulsory licensing systems one that balances patent enforcement with health equity, cultural sovereignty, and sustainable trade. By integrating indigenous voices, refining legal mechanisms, and fostering cross-border collaboration, India and Asia can lead away for a just and inclusive pharmaceutical ecosystem, these established reforms would not only fulfil international obligations under the Doha Declaration and Nagoya Protocol but also position traditional medicines as a cornerstone of global healthcare justice.
REFERENCES
[1] ‘WTO’ (The South Centre) <https://www.southcentre.int/category/issues/trade-and-investment/wto/> accessed 15 March 2025
[2] Velasquez, G , ‘Intellectual Property, Public Health, and Access to Medicines in International Organizations’ (South Centre, Research Paper No. 78, 2017) <https://hdl.handle.net/10419/232196> accessed 12 March 2025
[3] ‘Access to Medicines and Intellectual Property’ (INTELLECTUAL PROPERTY, PUBLIC HEALTH AND ACCESS TO MEDICINES IN INTERNATIONAL ORGANIZATIONS) <https://www.southcentre.int/wp-content/uploads/2013/05/RP47_WTO-role-in-IP-and-access-to-medicines_EN.pdf> accessed 15 March 2025
[4] Bayer Corporation v Union of India WP No 1323 of 2013 [15 July 2014] (Bom HC)
[5] ‘Tkdltraditional Knowledge Digital Library’ (TKDLTraditional Knowledge Digital Library) <https://www.tkdl.res.in/ > accessed 15 March 2025
[6] World Health Organization, ‘WHO Traditional Medicine Strategy: 2014-2023’ (2013) <https://iris.who.int/bitstream/handle/10665/92455/9789241506090_eng.pdf> accessed 12 March 2025
[7] ‘The State Council Information Office of the People’s Republic of China’ (Traditional Chinese medicine in China)
<http://english.www.gov.cn/archive/white_paper/2016/12/06/content_281475509333700.htm> accessed 15 March 2025
[8] ‘World Trade Organization’ (WTO)
<https://www.wto.org/english/tratop_e/trips_e/intel2_e.htm> accessed 15 March 2025
[9] CSIR, ‘Bio-piracy of Traditional Knowledge’ < http://www.tkdl.res.in> accessed 13 March 2025
[10] ‘London School of Hygiene and Tropical Medicine’ (1986) 60 Journal of Helminthology
[11] Bayer Corporation v Natco Pharma Ltd OA/35/2012/PT/MUM [4 March 2013] (IPAB)
[12] ‘Trade Related Aspects of Intellectual Property Rights (TRIPS)’ (BYJUS, 26 June 2023) <https://byjus.com/free-ias-prep/trade-related-aspects-of-intellectual-property-rights-trips/> accessed 10 April 2025
[13] Patents Act 1970 (India)
[14] Intellectual Property India. ‘Official website of Intellectual Property India’(n.d.). <https://ipindia.gov.in/> accessed 17 March 2025
[15] Correa, M, ‘Intellectual Property Rights, and the Use of Compulsory Licenses: Options for Developing Countries’ (Trade-Related Agenda, Development and Equity Working Paper, South Centre, Geneva 1999)
[16] World Health Organization, ‘TRIPS Agreement and its Impact on Health’ (WHO 2006)
[17] Patents Act 1970 (India)
[18] World Trade Organisation, ‘Regional trade agreements’ <https://www.wto.org/index.htm> accessed 15 March 2025
[19] Convention on Biological Diversity, ‘Home’ (Convention on Biological Diversity) <https://www.cbd.int/ > accessed 18 April 2025
[20] Armouti, W and Naser, M , ‘Traditional knowledge and patent protection: conflicting views on international patent standards’ (2010)
32(3) EIPR <https://pubmed.ncbi.nlm.nih.gov/23986896> accessed 15 March 2025
[21] ‘WIPO – World Intellectual Property Organization’ (WIPO – World Intellectual Property Organization) <https://www.wipo.int/> accessed 17 March 2025
[22] Patents Act 1970 (India)
[23] Patents Act 1970 (India)
[24] E, Urias and SV, Ramani ‘Access to Medicines after Trips: Is Compulsory Licensing an Effective Mechanism to Lower Drug Prices? A Review of the Existing Evidence’ (Journal of International Business Policy, 2020) <https://pmc.ncbi.nlm.nih.gov/articles/PMC7468182/> accessed 18 April 2025
[25] Thai Patent Act (1979)
[26] Patent Law 1984 (China)
[27] Patent Law 1984 (China)
[28] Thiru, ‘WTO Trade Policy Review: Malaysia Explains Its Compulsory License for Hep C Drug’ (Knowledge Ecology International, 27 April 2018) <https://www.keionline.org/27688> accessed 18 April 2025
[29] ‘LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].’ (National Centre for Biotechnology Information) <https://www.ncbi.nlm.nih.gov/books/> accessed 18 April 2025
[30] Paliwal, A, ‘Indian company gets licence to manufacture and sell patented cancer drug’(DownToEarth,2012)<https://www.downtoearth.org.in/environment/indian-company-gets-licence-to-manufacture-and-sell-patented-cancer-drug-37393> accessed 15 March 2025
[1] ‘WTO’ (The South Centre) <https://www.southcentre.int/category/issues/trade-and-investment/wto/> accessed 15 March 2025.
[2] G Velasquez, ‘Intellectual Property, Public Health, and Access to Medicines in International Organizations’ (South Centre, Research Paper No. 78, 2017) <https://hdl.handle.net/10419/232196> accessed 12 March 2025.
[3] ‘Access to Medicines and Intellectual Property’ (INTELLECTUAL PROPERTY, PUBLIC HEALTH AND ACCESS TO MEDICINES IN INTERNATIONAL ORGANIZATIONS) <https://www.southcentre.int/wp-content/uploads/2013/05/RP47_WTO-role-in-IP-and-access-to-medicines_EN.pdf> accessed 15 March 2025.
[4] Bayer Corporation v Union of India WP No 1323 of 2013 [15 July 2014] (Bom HC).
[5] ‘Tkdltraditional Knowledge Digital Library’ (TKDLTraditional Knowledge Digital Library) <https://www.tkdl.res.in/ > accessed 15 March 2025.
[6] World Health Organization, ‘WHO Traditional Medicine Strategy: 2014-2023’ (2013) <https://iris.who.int/bitstream/handle/10665/92455/9789241506090_eng.pdf> accessed 12 March 2025.
[7] ‘The State Council Information Office of the People’s Republic of China’ (Traditional Chinese medicine in China) <http://english.www.gov.cn/archive/white_paper/2016/12/06/content_281475509333700.htm> accessed 15 March 2025.
[8] ‘World Trade Organization’ (WTO) <https://www.wto.org/english/tratop_e/trips_e/intel2_e.htm> accessed 15 March 2025.
[9] CSIR, ‘Bio-piracy of Traditional Knowledge’ < http://www.tkdl.res.in> accessed 13 March 2025.
[10] ‘London School of Hygiene and Tropical Medicine’ (1986) 60 Journal of Helminthology 14.
[11] Bayer Corporation v Natco Pharma Ltd OA/35/2012/PT/MUM [4 March 2013] (IPAB).
[12]‘Trade Related Aspects of Intellectual Property Rights (TRIPS)’ (BYJUS, 26 June 2023) <https://byjus.com/free-ias-prep/trade-related-aspects-of-intellectual-property-rights-trips/> accessed 10 April 2025.
[13] Patents Act 1970 (India), s 3(p).
[14] Intellectual Property India, ‘Official website of Intellectual Property India’(n.d.). <https://ipindia.gov.in/> accessed 17 March 2025.
[15] Carlos M Correa, ‘Intellectual Property Rights, and the Use of Compulsory Licenses: Options for Developing Countries’ (Trade-Related Agenda, Development and Equity Working Paper, South Centre, Geneva 1999).
[16] World Health Organization, ‘TRIPS Agreement and its Impact on Health’ (WHO 2006) 7, 33.
[17] Patents Act 1970 (India), s 3(d).
[18] World Trade Organisation, ‘Regional trade agreements’ <https://www.wto.org/index.htm> accessed 15 March 2025.
[19] Convention on Biological Diversity, “Home” (Convention on Biological Diversity) <https://www.cbd.int/ > accessed 18 April 2025.
[20] Wael Armouti and Mohammad Naser, ‘Traditional knowledge and patent protection: conflicting views on international patent standards’ (2010) 32(3) EIPR 119 <https://pubmed.ncbi.nlm.nih.gov/23986896> accessed 15 March 2025.
[21] “WIPO – World Intellectual Property Organization” (WIPO – World Intellectual Property Organization) <https://www.wipo.int/> accessed 17 March 2025.
[22] Patents Act 1970 (India), s 84.
[23] Patents Act 1970 (India), s 92.
[24] Urias E and Ramani SV, ‘Access to Medicines after Trips: Is Compulsory Licensing an Effective Mechanism to Lower Drug Prices? A Review of the Existing Evidence’ (Journal of International Business Policy, 2020) <https://pmc.ncbi.nlm.nih.gov/articles/PMC7468182/> accessed 18 April 2025.
[25] Thai Patent Act B.E. 2522 (1979).
[26] Patent Law 1984 (China), art 50.
[27] Patent Law 1984 (China), art 49.
[28] Thiru, ‘WTO Trade Policy Review: Malaysia Explains Its Compulsory License for Hep C Drug’ (Knowledge Ecology International, 27 April 2018) <https://www.keionline.org/27688> accessed 18 April 2025.
[29] ‘LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet].’ (National Centre for Biotechnology Information) <https://www.ncbi.nlm.nih.gov/books/ > accessed 18 April 2025.
[30] Ankur Paliwal, ‘Indian company gets licence to manufacture and sell patented cancer drug’(DownToEarth,2012) <https://www.downtoearth.org.in/environment/indian-company-gets-licence-to-manufacture-and-sell-patented-cancer-drug-37393> accessed 15 March 2025.
